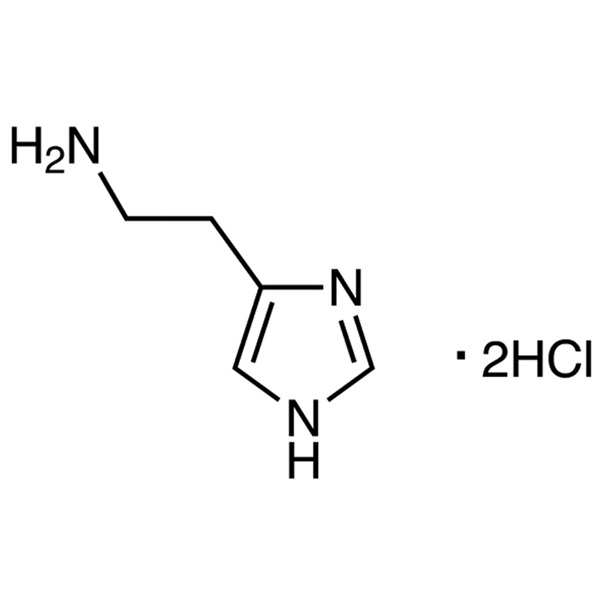
Histamine Dihydrochloride CAS 56-92-8 Assay 98.5~101.0% (Titration) Factory
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Histamine Dihydrochloride (CAS: 56-92-8) with high quality. Raw material L-Histidine (H-His-OH) (CAS: 71-00-1) We can provide worldwide delivery, small and bulk quantities available. If you are interested in Histamine Dihydrochloride, Please contact: alvin@ruifuchem.com
| Chemical Name | Histamine Dihydrochloride |
| Synonyms | Histamine 2HCl; Histamine DiHCl; 2-(4-Imidazolyl)ethylamine Dihydrochloride; 2-(1H-Imidazol-4-yl)ethylamine Dihydrochloride; 2-(1H-Imidazol-4-yl)ethylamine Dihydrochloride; 1H-Imidazole-4-ethanamine Dihydrochloride; Ceplene; Peremin |
| Stock Status | In Stock, Production Capacity 20 Tons per Month |
| CAS Number | 56-92-8 |
| Molecular Formula | C5H9N3·2HCl |
| Molecular Weight | 184.06 |
| Melting Point | 247.0~249.0℃(lit.) |
| Density | 1.14 |
| Sensitive | Hygroscopic |
| Water Solubility | Completely Soluble in Water, Almost Transparency |
| Solubility | Very Soluble in Methanol, Soluble in Ethanol, Insoluble in Ether |
| Storage Temp. | Sealed in Dry, Store at Room Temperature |
| COA & MSDS | Available |
| Brand | Ruifu Chemical |
| Hazard Codes | Xn,Xi | RTECS | MS1575000 |
| Risk Statements | 36/37/38-42/43-42-22 | F | 1-8-9 |
| Safety Statements | 22-26-36/37/39-45-37-36/37 | TSCA | Yes |
| RIDADR | 3335 | Hazard Class | Irritant |
| WGK Germany | 2 | HS Code | 2933990099 |
| Items | Inspection Standards | Results |
| Appearance | White Crystals or Crystalline Powder | Conforms |
| Identification | Infrared Absorption Spectrum | Conforms |
| Appearance of Solution | Clear | Pass |
| Histidine (TLC) | Not be Detected | Conforms |
| Sulfate (SO4) | ≤0.020% | <0.020% |
| Iron (Fe) | ≤10ppm | <10ppm |
| Heavy Metals (Pb) | ≤10ppm | <10ppm |
| Arsenic (As2O3) | ≤10ppm | <10ppm |
| Loss on Drying | ≤0.50% | 0.24% |
| Sulfated Ash | ≤0.20% | 0.06% |
| Assay | 98.5 to 101.0% (by Titration) | 99.36% |
| pH Value | 2.85 to 3.60 (10% aq. Solution) | 3.16 |
| Conclusion | Accords with the Standard of AJI97, EP9.0 | |
| Main Uses | Amino Acids; Food Additives; Pharmaceutical Intermediates | |
Raw Materials and Solvents Involved in the Synthesis Process
Raw materials:
L-Histidine (H-His-OH) CAS 71-00-1
Acetophenone CAS 98-86-2
Hydrochloric Acid CAS 7647-01-0
Solvents
Diethylene Glycol CAS 111-46-6
Ethanol CAS 64-17-5
Isopropyl Alcohol CAS 67-63-0
Water
Histamine Dihydrochloride (CAS: 56-92-8) EP9.0 VolumeⅡTest Method
DEFINITION
Histamine dihydrochloride contains not less than 98.5 percent and not more than the equivalent of 101.0 percent of 2-(1H-imidazol-4-yl)ethan-1-amine dihydrochloride, calculated with reference to the dried substance.
CHARACTERS
A white or almost white, crystalline powder or colourless crystals, hygroscopic, very soluble in water, soluble in alcohol.
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with histamine dihydrochloride CRS. Examine as discs prepared using 1 mg of substance.
B. Examine the chromatograms obtained in the test for histidine. The principal spot in the chromatogram obtained with test solution (b) is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve 0.1 g in 7 mL of water R and add 3 mL of a 200 g/L solution of sodium hydroxide R. Dissolve 50mg of sulfanilic acid R in a mixture of 0.1 mL of hydrochloric acid R and 10 mL of water R and add 0.1 mL of sodium nitrite solution R. Add the second solution to the first and mix. A red colour is produced.
D. It gives reaction (a) of chlorides (2.3.1).
TESTS
Solution S. Dissolve 0.5g in carbon dioxide-free water R prepared from distilled water R and dilute to 10 mL with the same solvent.
Appearance of solution. Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2,Method II).
pH (2.2.3). The pH of solution S is 2.85 to 3.60.
Histidine. Examine by thin-layer chromatography (2.2.27), using a TLC silica gel G plate R.
Test solution (a). Dissolve 0.5g of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Test solution (b). Dilute 2 mL of test solution (a) to 10 mL with water R.
Reference solution (a). Dissolve 0.1 g of histamine dihydrochloride CRS in water R and dilute to 10 mL with the same solvent.
Reference solution (b). Dissolve 50 mg of histidine monohydrochloride R inwater R and dilute to 100 mL with the same solvent.
Reference solution (c). Mix 1 mL of test solution (a) and 1 mL of reference solution (b).
Apply to the plate 1 μL of test solution (a), 1 μL of test solution (b), 1 μL of reference solution (a), 1 μL of reference solution (b) and 2 μL of reference solution (c). Develop over a path of 15cm using a mixture of 5volumes of concentrated ammonia R, 20 volumes of water R and 75 volumes of acetonitrile R. Dry the plate in a current of air. Repeat the development in the same direction, dry the plate in a curren of air and spray with ninhydrin solution R1. Heat the plate at 110°C for 10 min. Any spot corresponding to histidine in the chromatogram obtained with test solution (a) is not more intense than the spot in the chromatogram obtained with reference solution (b) (1 per cent). The test is not valid unless the chromatogram obtained with reference solution (c) shows 2 clearly separated spots.
Sulfates (2.4.13). 3mL of solution S diluted to 15 mL with distilled water R complies with the limit test for sulfates (0.1 percent).
Loss on drying (2.2.32). Not more than 0.5 percent, determined on 0.20 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14). Not more than 0.1 percent, determined on 0.5 g.
ASSAY
Dissolve 0.080 g in a mixture of 5.0 mL of 0.01 M hydrochloric acid and 50 mL of alcohol R. Carry out a potentiometric titration (2.2.20), using0.1 M sodium hydroxide. Read the volume added between the first and third points of inflexion.
1mL of 0.1 M sodium hydroxideis equivalent to 9.203 mg of C5H11Cl2N3.
STORAGE
Store in an airtight container, protected from light.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Application of Histamine Dihydrochloride (CAS: 56-92-8)
1. Histamine Dihydrochloride has been shown to activate nitric oxide synthetase and suppress or inhibit the generation of reactive oxygen species (ROS). Inhibition of ROS by Histamine Dihydrochloride allows activation of T cells and NK cells by IL-2. In a rat model, histamine dihydrochloride suppressed ROS generated by Kupffer cells through the H2 histamine receptor. It is a potent vasodilator and endogenous histamine receptor agonist.
2. Histamine Dihydrochloride (trade name Ceplene) is a salt of Histamine which is used as a drug for the prevention of relapse in patients diagnosed with acute myeloid leukemia (AML).
It is an FDA approved active ingredient for topical analgesic use and is available in such products as Australian Dream Cream, which is used for the temporary relief of minor aches and pains of muscles and joints associated with arthritis, simple backache, bruises, sprains, and strains.
3. Histamine Dihydrochloride is used for sustained remission and prevention of relapse in adult patients with acute myeloid leukemia (AML) following initial remission therapy. The drug reduces the production of oxygen groups by autophagocytes, inhibits nicotinamide adenine dichemicalbo phosphate oxidase and prevents interleukin-2 from activating NK cells and T cells. Histamine Dihydrochloride injection was approved on the basis that its complete remission in combination with interleukin-2 significantly reduced relapse in AML patients.